 With the world’s major news organizations now focused on the horrific sexual side effects of Propecia, more evidence is coming forward that the Merck manufactured drug can affect the brain.
With the world’s major news organizations now focused on the horrific sexual side effects of Propecia, more evidence is coming forward that the Merck manufactured drug can affect the brain.
AdverseEvents, Inc., the leading resource for information on drug side effects, released a report earlier this year identifying FDA approved medications that are most associated with causing brain-related side effects.
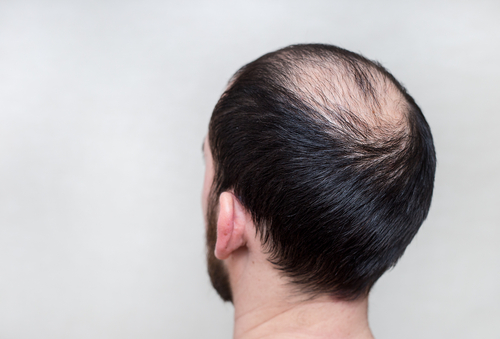
By a wide margin, the top drug linked to side effects involving loss of libido was Propecia followed by Proscar. Propecia is the brand name for the drug finasteride when it is prescribed in 1mg doses to treat male pattern baldness. (When prescribed in 5mg doses to treat enlarged prostate it is sold under the brand name Proscar).
- Murali Doraiswamy, M.D., a professor and leading neuropsychiatric drug safety researcher at Duke University Medical Center said, “While the warning signals in this report don’t necessarily prove a causal relationship, they are often the first sign of such a link.”
The Duke Professor continued by stating that, “It is critical for consumers and prescribers to be aware of such potentially adverse effects. Some of these side effects, such as loss of libido or amnesia, can have a devastating impact on a person’s quality of life.”
Brian Overstreet, president of AdverseEvents said, “While the data does have limitations, the ability to easily and quickly mine critical data using our proprietary system provides a significant step forward to a better understanding of real-world side-effect risks, the enabling of more accurate safety signaling, and the propelling of further clinical evaluation of potential problems.”
The company used its reporting system to determine the top drugs in several side-effect categories. Case reports from 1/1/04 – 9/30/2011 were studied and drugs with limited case reports were excluded from the final list.
When it came to the top mind-altering drugs linked to loss of libido, Propecia ranked first by a wide margin.
With AEI, the healthcare industry is able to quantify the benefit-risk assessments of FDA approved drugs to fully understand the scope of safety issues, based on accurate rates of side effects from such medications.
As previously reported by the Examiner, the FDA urges anyone affected by Propecia to “formally document your case via the MedWatch reporting system. The information you provide will be added to FDA’s post-marketing safety database and reviewed by the post marketing safety staff.



